Stem Cell Therapy for Osteoarthritis
Story by Heather Smith ThomasPhotos provided by Karen Mantel, Ontario Veterinary College, University of Guelph and Dr. Erin Roberts, University of Calgary

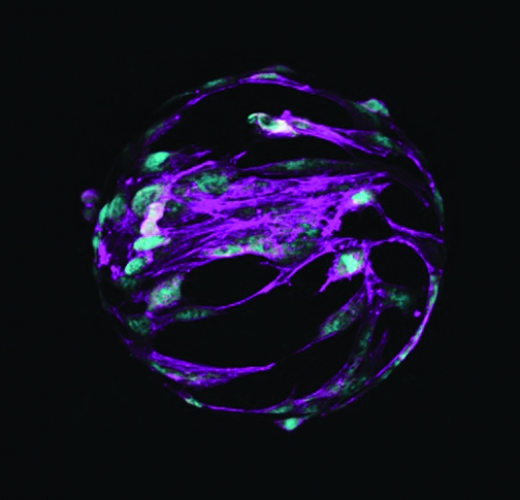
Stem cell therapy has been utilized in horses to help heal tendon, ligament and joint injuries for more than 20 years, and new uses are continually explored. Stem cells used in horses are mesenchymal stromal cells (MSCs) which are isolated from fetuses, foals or adult horses, as opposed to embryonic stem cells isolated from embryos. The MSC equine stem cells can be isolated from almost any tissue but most commonly obtained from bone marrow, fat tissue and from the umbilical cord of newborn foals.
The use of certain stem cells requires a laboratory culture to isolate them and expand their numbers. These cells are characterized by their ability to become other types of cells in the lab and to modify the function of cells from the immune system such as lymphocytes. Cells from bone marrow and fat tissue are also used after a brief laboratory processing step where all the cells are concentrated from the sample without a culture to select and expand MSCs. These processes are known as bone marrow concentrate or stromal vascular fraction, depending on whether the starting material is bone marrow aspirate or fat tissue. Sometimes these cell preparations are referred to as stem cell treatments although very few of these cells would be MSCs.
Autologous versus allogeneic use of cells is another important distinction. Autologous cells are from the patient itself. Allogeneic cells are from a donor horse and are placed in other horses. The advantage of using autologous cells is that they are not rejected by the patient’s immune system, and there are less regulatory concerns with their use. Disadvantage of using autologous MSCs is that it takes 2-3 weeks to isolate and expand the cells prior to use, to get enough of them. This involves a two-step process requiring the horse to return for treatment--after diagnosis and initial sample collection.
This may hamper optimal treatment time since an adequate number of cells are not readily available. Allogeneic use of cells has the advantage of making cells readily available and provides time to select and potentially enhance cell functions prior to use.
The disadvantage is that these cells (from another horse) are recognized by the patient’s immune system (and rejected) faster than autologous cells. Also, the regulations are different; allogeneic cell product development is considered drug development. Often autologous cells are provided for use at chilled temperatures whereas allogeneic cells are often provided frozen and thawed immediately prior to use. The thawing process can influence the utility of the cells.
Today, most commercial strategies are focused on developing frozen allogeneic cell products. One approach is the use of allogeneic stem cells from umbilical cord blood that could be basically off-the-shelf/storable and given to any horse.
The Challenges Of Treating Osteoarthritis
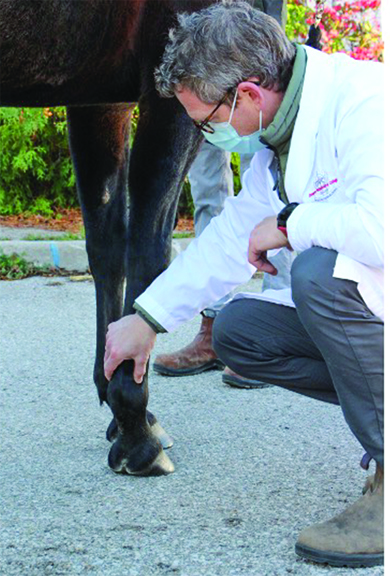
There are still a number of conditions in horses and humans that are difficult to treat successfully; we don’t have good treatments for those and/or the treatments are not 100% successful. Osteoarthritis (OA) is one of those conditions for which we don’t have good treatments. Dr. Thomas Koch (Department of Biomedical Sciences, Ontario Veterinary College) has been working with stem cells for many years and says the fact we don’t have good treatments for OA is reflected by the many different ways that horses with joint pain are being managed and treated.
“There are many products being used for patients with OA, and veterinarians have different preferences in treatment—based on personal experience, and the experience of their mentors. This in itself indicates that no one treatment has been shown to be superior. There is interesting data in humans and various veterinary species, however, showing that stem cells may be useful in treating some joint conditions,” he says.
Koch is founder and CEO of eQcell, a company that is currently starting two blinded, randomized trials in equine synovitis and early stages of osteoarthritis--one at the University of Guelph’s Ontario Veterinary College Equine Sports Medicine and Reproductive Centre, and the other trial at the University of California-Davis Veterinary Institute for Regenerative Cures (VIRC). The Canadian study in equine fetlock and carpal joint osteoarthritis is authorized by Health Canada’s Veterinary Drug Directorate and is the first stem cell trial in Canada for treatment of equine osteoarthritis. The U.S. study in fetlock osteoarthritis is being conducted under VIRC’s Investigational New Animal Drug (INAD) with the FDA.
“Part of the challenge in treating OA is that even though we talk about it as though it is one entity, in human medicine we are starting to recognize that there may be up to 100 different underlying mechanisms that more or less manifest the same way. We can look at these problems more closely now, with new molecular technologies. Now we know that OA is just a term for a very large category of conditions,” he says.
This helps explain why some treatments work and some don’t. “It’s partly chance whether or not the treatment you provide matches that particular underlying condition. There are obviously a lot of issues, with some horses responding to treatment and some not. We realize that OA is a disease complex--more than just one disease,” Koch says.
Often these horses are being managed with daily medication to try to ease their pain, if they are not participating in a sport that prohibits drug use while competing. Pain management can be a challenge for horses that must be drug-free during competitions. A retired horse or a recreational horse might get by with a little “bute” every day, but a sport horse or race horse cannot be medicated in that manner.
“Another drawback is that NSAIDs like bute have a narrow therapeutic index (a small window of effectiveness that is not very far from toxic levels). A little too much, and they cause problems or gut issues. It’s a fine line. In an older, geriatric horse, for instance, those drugs may not be metabolized the same as in a younger horse, and the therapeutic index is even smaller.” A person must be careful to not overdose the horse.
Every time you rely on daily medication, there can also be issues with consistency and compliance. “In human medicine, 60% of treatment failures are due to people not taking the medications prescribed, because it’s a hassle or they forget. They take their medication for a while in the beginning and feel better, and then stop taking it because they either forget about it or feel they don’t need it anymore, and then the arthritis flares up again. You don’t have consistent management of the condition when taking the medication sporadically,” he explains.
“It’s the same for many horses. People put them on certain drugs, and they get better, then the owner stops the medication, and the horse gets painful again. The pain is not always managed consistently.” Also, these drugs merely aid pain management and don’t facilitate healing or resolution of the condition. The underlying process is still progressing, and joint damage continues.
There are a number of biologic products that can be injected into joints, like PRP (platelet rich plasma) or IRAP (interleukin receptor antagonist protein). Interleukins are mediators of inflammation like histamine and other cytokines that cause bad things to happen in injured tissue.
“The advantage with these biologic treatments is that there have been fewer regulations regarding their use (as opposed to stem cells). But again, it can be hit or miss in whether they work, depending on the individual horse and how well it responds. IRAP is a very targeted treatment against interleukin-1 but if the pain is not driven by an underlying interleukin-1 inflammation, it will be less successful,” says Koch.
Steroids have also been used for treating OA, but there is controversy about using those in joints; some people feel that these can damage the cartilage, so they try to avoid steroids. There are also different types of steroids that influence the joint in different ways. “Steroid treatments help some horses, but may not be ideal, and there are some issues regarding their use, and concerns related to the long-term health of the joint.”
Advantages Of Stem Cells
Stem cells therapies are gaining interest because they have several advantages over other types of treatment. “Veterinarians have been using cell-based therapies in dogs for joint treatment. There are not a lot of good studies yet—but there are many dogs that receive preparations containing cells that seem to help. Some of these are preparations with fast turnaround (stromal vascular fraction or bone marrow concentrate) where they use cells from the fat tissue or bone marrow in dogs. Others are culture expanded MSCs. Both autologous and allogeneic cells have been used. The results are all over the place; some animals seem to respond very well. Some dogs receive an injection and are good for a year, but some do not respond. I think this has to do with the disease complex and also the fact that these new therapies are often reserved for the worst cases. Stem cells might not be used until the veterinarian has tried everything else—so it may or may not work in those cases,” he explains.
“This is typical; if veterinarians are not familiar with the therapy, they don’t want to do any harm and may reserve cell therapies for those last-ditch effort cases—and it is hard to judge efficacy in those cases. A person might realize they are safe (if the treatment doesn’t harm the patient) but it may be hard to judge the efficacy because the disease is already at such an end stage,” Koch says.
“There are now two cell products approved in Europe, for treating non-septic inflammatory joint pain in horses caused by synovitis and early OA. These are HorStem and Arti-Cell Forte. These products are both using culture expanded MSCs. HorStem (a product from Spain) utilizes cells from equine placental umbilical cord tissue, and Arti-Cell Forte isolates MSCs from the peripheral blood of adult horses. These two products are both approved for use in horses with joint pain due to early-stage synovitis. They are slightly different in formulation.
“HorStem contains only the cells (15 million stem cells) and Arti-Cell Forte is a combination product. It is often portrayed by the company as a stem cell product but is actually only 2 million MSCs, plus PRP (platelet rich plasma). So, with that product it’s a bit difficult to know if the perceived effect is due to the cells or the PRP, or whether the two may have a synergistic effect working together. I haven’t seen any studies where the company treated horses with 2 million cells alone, the PRP alone, or the combination, to compare or show that the combination was superior to either one alone. That would be interesting to know,” says Koch. Maybe a study like that will be done, now that these products are commercially available.
“A study at Cornell University investigated mesenchymal stem cells from horses in their research animal model, in which they damaged the cartilage in the joints of research horses. Some of those joints got stem cells and some joints got just the injection vehicle/fluid without cells. The joints were all treated at the same time. In the follow-up, the researchers saw that the cartilage in the joints that received the cell formulation were much more preserved than the joints that did not get those cells.”
There was less cartilage damage in the joints that received the cells. “This is a step toward the concept of regeneration. This field has been called regenerative medicine, but we have to be careful in this kind of thinking because it implies that the therapies, we’re using are rolling the marble up the hill and back toward an earlier, healthier tissue state. I am not sure if that’s true,” he says.
“I am not sure if these therapies can turn a damaged joint into a less-damaged joint, but the study at Cornell indicates that maybe we can arrest the damage and prevent further damage. These are two very different situations. We can maybe freeze the joint in time, and it won’t get worse. We might be able to have some degree of disease arrest and joint preservation, but that’s not the same as a joint being regenerated and looking more like a normal joint that never had injury.”
In equine tendon injuries there are many treatments that do more than arrest the damage, because there is some tissue healing. “Still, if you do histology on that tendon, you can see that it’s not normal, original tendon tissue. It’s enhanced repair. With tendons and ligaments, the tissue can repair itself and become somewhat elastic and strong again, compared to scar tissue,” says Koch.
“If a horse has a tendon injury and you simply leave it alone, it will form strong scar tissue, but the scar tissue is like a knot in a rubber band. The knot itself is strong, but the interface above and below the knot is weak. This is typically where re-injury occurs. The concern with a tendon injury is that it will happen again—and when it does, it’s almost always between the scar tissue and the normal tendon tissue. Some people would say the scar tissue is too strong, and not flexible; when the tendon needs to stretch, there’s no give. People think the reason that the risk of re-injury of tendons and ligaments after stem cell treatment is reduced by 50% is because the repaired tissue is more flexible and giving—functioning more like the original tendon,” he explains.
“This is great, but we still can’t call it regenerative. We don’t want to create false expectations. I am excited about the use of stem cells. I think they will prove to have a role to play, but we need realistic expectations. With joint disease, there are strong indications that these cells may be disease-modifying, and potentially arrest disease progression. But to determine this, we’d have to damage the joint, let the damage progress for a while and then treat some of the joints with the cells and some with the placebo. You’d examine the joints at that time, to have a base line, to then be able to see later if the cells arrested the damage and preserved the cartilage at the damaged state or made it look better than it did at the time of treatment. If that were the case, then I would say it had some regenerative qualities, but those studies have not yet been done.”
It is exciting that two new products in Europe have been approved; he feels they will benefit horses. “One of those companies was acquired by Boehringer Ingelheim (a large international pharmaceutical company) last summer, stepping into this field of veterinary regenerative medicine. This is a boost for everyone, to know that this big company thinks stem cells and regenerative medicine have a role to play in veterinary medicine, going forward,” says Koch.
The resources and enthusiasm that come with that will direct more money into this field. “I think we will see this type of therapy become available. Some of the advantages of doing this in equine medicine is that joint injections are common; equine veterinarians are comfortable doing this. Small animal veterinarians are less comfortable injecting cat and dog joints; it’s more of a specialist treatment.”
There’s possibility now for more consistent disease management—maybe being able to use infrequent joint injections to keep a horse comfortable. “It might only have to be every 6 months or every 8 months or even longer. It might depend on the horse; there may be some horses that don’t respond to the treatment. But for horses that do respond, we may be able to manage their disease more consistently and have some degree of joint preservation—and disease arrest,” he says. This could be potentially career-extending, for some horses.
“There is some interesting data now, both in veterinary medicine and human medicine, and the fact that there are two products approved in Europe is exciting. Our company, eQcell, is trying to do something similar with products that are currently available. We have trials starting in the U.S. at UC-Davis. The lead investigator there is Dr. Larry Galuppo. The trial at the Ontario Veterinary College in Canada is led by Dr. Judith Koenig (Equine Sports Medicine & Reproduction Centre). Both trials have approval—from Health Canada and from the FDA—since stem cells are regulated because they are considered drugs,” says Koch.
This is the reason there are no approved stem cell products currently available in North America. Other regenerative therapies like IRAP and PRP fall under the FDA’s category of devises rather than drugs. “There is a very big difference between getting a device approved by FDA and getting a drug approved. It is very expensive and time-consuming to get a drug approved, and this is a big impediment for veterinary medicine, because from a pharmaceutical perspective it is still a very small market,” he explains.
“When companies look at the market and the cost of getting a product developed and approved, it becomes difficult to make a financial case for it, even for products that would greatly benefit the horse,” he says.
“Our company will be pursuing the European market prior to the North American market because there is already a precedent in Europe (with two products approved) that we can follow, and the likelihood of getting approval is higher. So we hope to get into Europe first and then continue our animal studies to get approval in North America. Equine veterinarians in North America would like to get access to some of the products in Europe. They are looking for alternatives for managing synovitis and early OA; there is still an unmet need and a desire to try these new products.”
The stem cell product that eQcell will be marketing will be allogeneic cells (from other horses rather than from the patient’s own body). “There are obvious advantages in using autologous cells from the patient; there are no concerns about immune recognition (and rejection of the cells). We do provide that service to equine veterinarians and have been doing that for the past 10 years. They take a bone marrow aspirate from the patient, ship it to us, and we isolate the cells from that—and ship it back two weeks later. This is just a small service business, however,” he says. It can’t help as many horses.
“Some veterinarians are also not comfortable taking a bone marrow aspirate to send to us. It’s also inconvenient for the horse owner and veterinarian because the horse has to come back two weeks later for treatment. This adds cost, and you are not in control of treatment timing; you are hostage to the laboratory schedule.”
By contrast, if you can use a product off the shelf, it can be given to the horse at the desired time of treatment, when you think treatment will be most helpful. “If the veterinarian diagnoses the horse and thinks it could benefit from stem cells, and has those cells in the clinic, it can be done right away. If it’s a referral hospital, sometime those horses are brought from far away. It’s not very convenient to have them come back later, with another long trip. This is partly why use of allogeneic cells is becoming more popular; they are much more convenient,” says Koch.
How The Cells Are Harvested And Grown
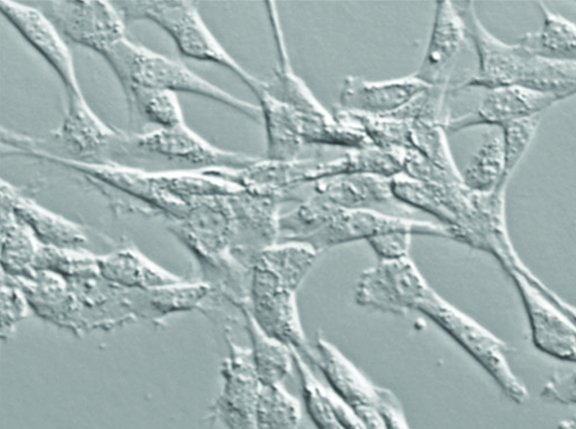
“The cells we are working with are from the umbilical cord blood of newborn foals. When I did my PhD work, I reported on the presence of these cells. This had been reported previously in humans, but we were the first to investigate whether we could find similar cells in equine umbilical cord blood, and we did. We gave that report in 2007,” he says.
“I finished my PhD and continued to work on these cells. Our company gets the cells from umbilical cord blood. Most other people get it from bone marrow or fat tissue. In Europe, one product (HorStem) contains cells from the umbilical cord tissue (not the blood) and the other product (Arti-Cell Forte) contains cells from the peripheral blood of adult horses--isolating cells from the blood, using my initial protocol for umbilical cord blood, since these also work for adult blood,” he explains.
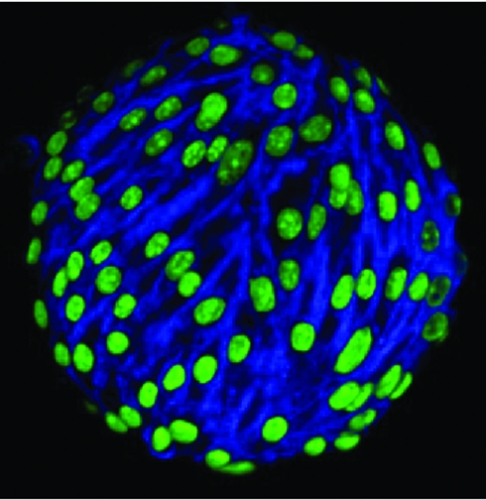
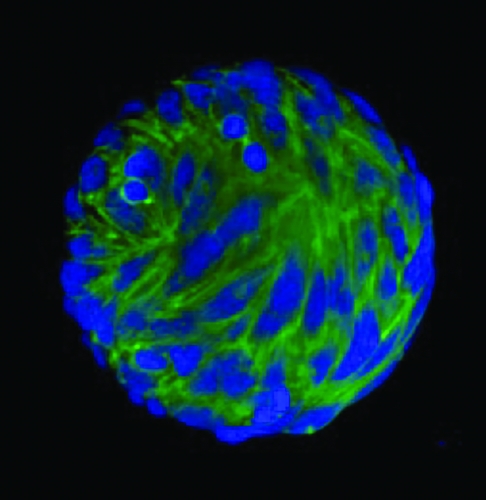
“These cells are characterized by their ability to adhere to certain types of plastics. In the lab we use plastic culture dishes and receive the cells into those. In the first few days a lot of cells are just floating in the media and don’t attach, and then some start attaching to the plastic. When we replace the media and aspirate it off and replace it with fresh media, this gets rid of all the floating cells. We end up with a cell population stuck to the plastic and the media is clear; there are no more floating cells because we have diluted them out,” says Koch.
After eight to 12 days some of the cells on the dish start to form colonies and expand and undergo cell division. “We use enzymes to lift them off the plastic; the enzymes cleave the binding between the cell and the plastic without damaging the cells. We harvest them this way and split them, putting them from one flask into three to five flasks of the same size. Those cells reattach and keep growing and propagating. We can eventually grow them into billions of cells,” he says.
The original cells from umbilical cord blood come from breeding farms. “I work with some high-end Standardbred and Thoroughbred farms in southern Ontario. During foaling season, they have eQcell’s collection kit at the farm, and whenever a foal is born, the attendant clamps the umbilical cord. Then when the foal stands up, the cord simply breaks at its natural breaking point. With the clamp across it (toward the mare side) the blood is not gushing out from the placenta through that break and is saved.”
Then the attendants use a blood transfusion bag to collect it. “These bags come preloaded from the company, with anticoagulant and a needle welded onto the end. The attendants simply clean the cord and put the needle into the blood vessel within it, and the blood can flow/drain into this collection bag. It’s non-invasive; you are not poking the mare or the foal—just saving the blood that would otherwise drain out and be lost.”
The infusion bag can be stored in a refrigerator overnight, then shipped by FedEx to the lab. “Even a day or two later, when it arrives at our lab, we can successfully isolate those cells. What’s nice about this source of cells is that they are as young as we can get them, and consistent. All our cells are from newborn foals, and mares that have no signs of disease during the pregnancy.”
There are advantages to having these very young cells. “If you get cells from fat (adipose) tissue or blood or bone marrow from adult horses, they may be somewhat damaged. All cells in the body become damaged as we get older. They age, so even if you have a healthy, normal animal, there are many stringent requirements for donor testing. Cells from an adult will be different,” he says.
With foal cells, the allogeneic cells have some variability between one foal and another, but these cells are still more consistent. “This is another advantage of allogeneic cells over autologous cells; we have the luxury of time, to work with these cells and apply different selection criteria. We can standardize and optimize our cell products because we don’t use every cell,” he explains.
By contrast, with autologous cells you get what you get. “They may grow well, or they may not, but you have no alternative. If you want to use them, you simply have to use what you get. They may not be optimal; maybe the horse has other conditions that affect the functionality of those cells. This is part of the problem with autologous cells, even though you don’t have immune issues. There may be other issues with those cells that affects their functionality.”
With allogeneic cells, it may be inconsistent in terms of whether horses develop antibodies against them. “So far there have been very few adverse reactions to them. We know these cells don’t stay around very long in the tissues, so they are getting recognized as foreign by the immune system and being removed by the body, as we would expect. But in spite of that, they seem to have a therapeutic effect,” he says.
The way they work is like a conductor of an orchestra. “The stem cell comes in and influences other cells in the body—in the joint or tissue. These cells are influenced by the stem cell and start to behave differently. Even though the stem cell gets removed by the immune system, there is a lasting effect in the tissue of the patient’s own cells behaving differently. This is probably why we see enhanced repair of those tissues. Some of those cells that are behaving differently are macrophages. They may be in an inflammatory state, but the stem cells communicate with those macrophages, and they switch and become an anti-inflammatory type of macrophage. These cells are not rejected by the immune system because they are the patient’s own cells. Those changed cells will stay around longer in the body and exert their therapeutic effects.”
The stem cells come in and have this echoing ripple effect throughout the tissue even after those stem cells have been removed. “In the future I think we will get better at selecting cells that have enhanced functionality for different conditions. We will be better at matching different types of cells with different disease conditions—so we can select the best possible formulations,” he says.